Reduction
According to the old (classical) concept, it is defined as the process which involves the addition of hydrogen or any other electro-positive element or radical to an element or a compound or the removal of oxygen or any other electronegative element or radical from a compound.
For example:
Addition of Hydrogen or any other electro-positive element.
- N2 +3H2 –> 2NH3
In this reaction H2 is added to N2 and is reduced to NH3.
- HgCl2 + Hg –> Hg2Cl2
Here, Hg (mercury), an electro-positive element, is added to HgCl2 and is thus reduced to Hg2Cl2.
Removal of oxygen or any other electro-negative element or radical.
- 2HgO –> 2Hg +O2
Here, HgO looses O2 and is reduced to Hg.
- 2FeCl3 + H2 –> 2FeCl2 + 2HCI
Here, FeCl3 looses Cl2, an electro-negative element and is reduced to FeCl2.
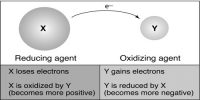
According to the electronic concept, reduction is a process which involves gain of electron by an atom. Due to gaining of electron there will be an increase of negative charge or decrease in positive charge.
Example:
- 2Na + Cl2 –> 2NaCl
In the above reaction chlorine gains one electron and is thus reduced.