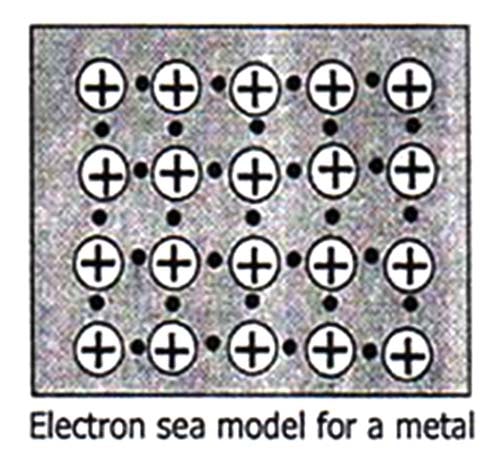
The force which binds together the atoms of metals is called metallic bond.
All the characteristics metallic properties can be explained on the basis of electron sea model as given below:
- Metallic Luster: The bright luster of metal is due to presence of delocalized mobile electrons which absorbs photon of light and start oscillating at a frequency equal to that of incident light. As a result these electrons emit radiation in the form of light due to which the incident light appears to be reflected and the surface acquires a shining appearance which is known as metallic luster.
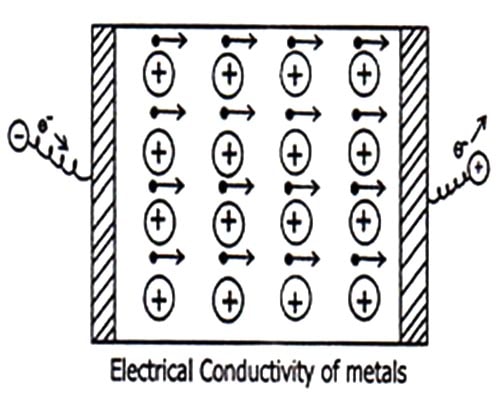
- Electrical Conductivity: When a potential difference is applied across the metal sheet, the free mobile electron starts moving towards positive electrons. These electrons are replaced by the electrons coming from the negative electrode. Thus the metallic sheet maintains the flow of electrons from negative electrode to positive electrode. Thus constitutes electrical conductivity.
- Thermal Conductivity: Thermal conductivity is also due to presence of mobile electrons whose kinetic energy increases on heating the metal. These energetic electron moves rapidly to the cooler parts and transfer their kinetic energy by means of collision with other electron. Therefore heat travels to cooler parts from hotter region.
- Malleability and Ductility: Metals can be beaten into sheets and drawn into wires. These properties exhibit in the metal due to non-directional nature of metallic bonds. Whenever any stress is applied on metals, the position of metallic kernel is altered without destroying the crystal. Due to slippage of the adjacent kernel from one part to another the metallic lattice gets deformed. Therefore, the environment of kernels does not change the deforming forces simply move the kernels from one lattice site to another.