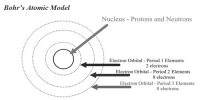
Aufbau is a German word which means building up. Aufbau’s principle states that electron goes to different orbitals according to their increasing energy. Electrons first go to the orbitals of lower energy and then gradually to orbitals of higher energy. It is also known as Building up Principle and (n+1) rule.
The orbitals which have a lower value of (n+1) has the lower energy and higher value of (n+1) has higher energy.
Example: For 4s orbital, n=4 and 1=0; (n+1) =4
For 3d orbital, n=3 and 1=2; (n+1) =5
Therefore electron will go to 4s orbital and then to 3d orbital.
If two orbitals have the same (n+1) value then the electron enters the orbital that has the lower principal quantum number such as-
For 3d orbital, n=3 and 1=2; (n+1) =5
For 4p orbital, n=4 and 1=1; (n+1) =5
So electron will go to 3d orbital first.
The electronic configuration according to Aufbau’s principal is given below,
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f