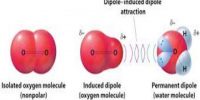
Diffusion:
The phenomenon by which the gases intermix with one another to form a uniform mixture when they are brought in contact, due to the random movement of gaseous molecules irrespective of their densities, is known as diffusion.
In other words, gaseous diffusion is the spontaneous intermixing of two or more gases of wide differences in their densities.
Effusion:
The process by which gases come out through a small hole (aperture) due to the effect of external pressure is called effusion.
Example: Ammonia gas is kept inside cylinder at a high pressure. If any hole remains in that ammonia cylinder then the ammonia will come out forcibly speed. This is effusion excluding external pressure, even though the gas if comes out then that is diffusion.