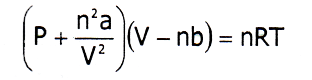Ideal Gas:
The gas which obeys all gas laws i.e. Boyle’s Law and Charle’s Law at all temperature and pressure is known as Ideal Gas.
Real Gas:
The gas which does not follow the gas laws i.e. Boyle’s Law and Charle’s Law at all temperature and pressure is called Real Gas. For example, H2, N2, O2 are real gases.
Difference between Ideal gas and Real Gas is as follow:
- Ideal Gas obeys all gas laws under all conditions of temperature and pressure.
- In the ideal gas the volume occupied by the molecules as compared to the total volume occupied by the gas it’s negligible.
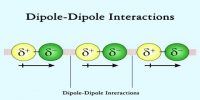
- The force of attraction among the molecules of the gas is negligible.
- It obeys ideal Gas equation PV=nRT.
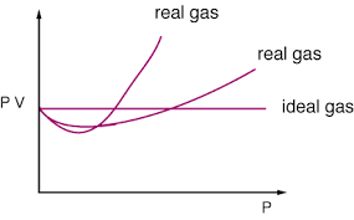
- Real Gas obeys gas laws only under low pressure and high temperature.
- In the real gas, the volume occupied by the molecules is not negligible as compared to the total volume occupied by the gas.
- The force of attraction among the molecules is not negligible at all temperatures and pressure.
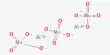
- It obeys Vander Waals Eqn: