Viscosity and Temperature
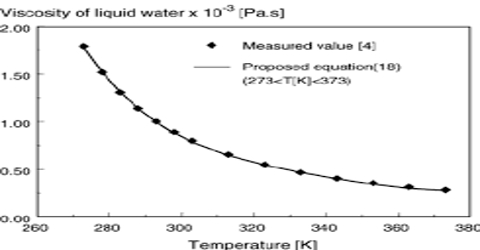
The viscosity of liquids usually decreases with increasing temperature. For many liquids the variation of viscosity with temperature may be represented by
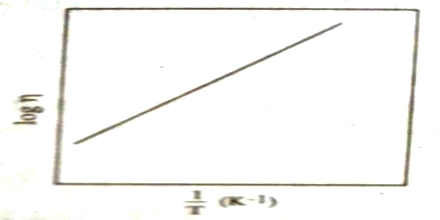
log η = A/T + B
Where A and B are constants for a given liquid (Figure). This is not, however, valid for all liquids.
Fig: log η plotted against 1/T
Viscosity measurements should, therefore, be carried out in constant temperature baths.
The viscosity of liquids increases as temperature decreases, whereas the viscosity of gases increases as temperature increases. Viscosity is a fluid’s confrontation to internal motion or flow and results from intermolecular resistance. With an increase in temperature there is usually an increase in the molecular interchange as molecules move faster in higher temperatures. So the viscosity of a gas will increase with temperature.