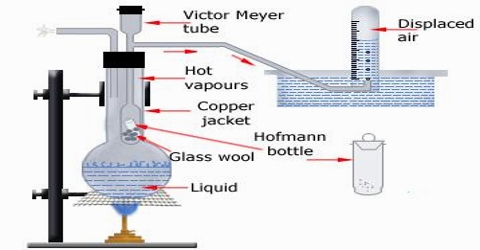
Victor Meyer’s method
This is perhaps the most common method for the determination of the molecular mass of substances which are liquid or solid at ordinary temperature. In this method the volume of a known mass of the substance is determined by measuring the volume of air displaced by it. The apparatus is shown in Figure 1.
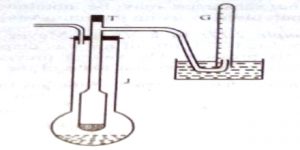
A long tube, usually made of glass, with a bulb at one end and a side tube near the open end, is the main part. This is known as the Victor Meyer tube, T, in which the substance is vaporized. This tube is surrounded by an outer jacket. J. made of glass or more conveniently, copper. The outer jacket contains some liquid which boils at a temperature 200 – 30°C higher than the experimental substance to be placed in the V.M. tube. There is an outlet of the packet to allow the escape of hot vapour. The side tube is bent as shown in Figure 2.22 and passes under water in a trough. A graduated tube, G, completely filled with water, is inserted over the open end of this side tube.
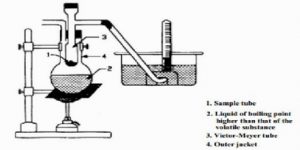
Fig 2: Victor Meyer’s apparatus
The V.M. tube is first dried thoroughly and a small quantity of dry sand or glasswool is placed at the bottom to prevent it from cracking when the bulb containing the experimental substance is allowed to fall into it. The tube is then placed in position and the liquid in the picket is boiled; the hot vapour heats the V. M. tube, air aside it expands and excess air bubbles come out through the end of the side tube, the end being under water.
While this is going on a bulb with drawn out ends is prepared from a piece of glass tubing, one end of the bulb being sealed and the longer end open. This is dried by holding over a flame, cooled and then tilled with a known quantity (0.1 g – 0.2 g) of the substance under investigation, the open end being sealed before the final weighing.
When no more air bubble escapes through the side tube of the V.M. tube, the temperature of the air in the inner tube is constant. The graduated tube is filled with water and placed over the side tube as shown in the diagram. The long stem of the bulb is broken at the tip, and then it is dropped into the V. M. tube through the top end and the tube Stoppard. These operations should be carried out as quickly as possible. The substance vaporizes immediately and the vapour displaces some of the air which is collected in the graduated tube. When all the displaced air has been Collected the graduated tube is removed and placed in a tall cylinder filled with water and the volume of the air noted after making the water level outside and inside the tube the same. The temperature of water in the tall jar and the barometric pressure are noted. The total volume of the air displaced is the volume which the vapour would occupy if it were possible to have it as a gas at the room temperature and the atmosphere pressure. It is not necessary to know the temperature of the bulb so long as we are sure that it is well above the boiling point of the experimental liquid. The pressure is corrected for the vapour pressure of water at the room temperature. The density of the vapour is calculated and hence the molecular mass of the substance may be obtained. The method can be used up to very high temperatures by using platinum vessels and electric heating.
Example: In a Victor Meyer experiment when 0.110 g of a substance was evaporated 27.0 mL of air u as displaced and collected over water at 27°C and 750 mm Hg pressure. The vapour pressure of water at this temperature is 25.1 mm Hg. Calculate the molecular mass of the substance.
Solution: The pressure of the gas in the tube;
(750-25.1)/760 = 0.954 atm.
The molecular mass; M = gRT/PV
= [(0.110) (0.082) (300.1)] / [0.954 (27/1000)] = 105