The great usefulness of thermodynamics to chemists can be summarized as follows:
- The laws of thermodynamics are not based on any assumption about the existence or nature of atoms, molecules or ions. Thus, if future experiments reveal that the accepted theories of the structure of atoms and molecules are inadequate, the laws of thermodynamics would still be valid.
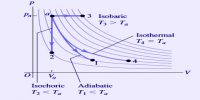
- It can predict the feasibility as well as direction of thermodynamic processes.
- It can explain why reactions can reach equilibrium position and what their compositions at equilibrium are.
- It can explain how reactions in electrochemical cells as well as in biological cells can be used to generate electricity.
- Applications of thermodynamic principles to colligative properties of solutions. Distribution Law, Phase Rule etc. led to the deductions of important relationships and better understanding at these and other phenomenon in physical sciences.
The principles of thermodynamics have few limitations as well. These are;
- Time is never a variable in thermodynamics. It does not give any indication of the rate of any process.
- The principles of thermodynamics are interested only in the initial and final states of the system. It does not give any information about how the final condition is attained or how long it takes to reach this condition. In other words, it does not give any idea about the mechanism of any process.
- Classical thermodynamics deals with macroscopic systems and does not give any idea about the role of individual atoms, molecules and ions on overall properties of a system.