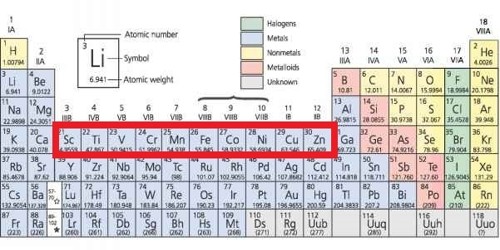
All transition elements are d-block elements, but all d-block elements do not transition elements.
D block elements are chemical elements having electrons filling to their d orbitals. All transition metals are d-block elements but all d-block elements are not transition elements because all d- block elements which don’t have completely filled d- orbitals are not counted as transition, so such elements are exceptional. Transition elements are chemical elements that have incompletely filled d orbitals at least in one stable cation they form. Example: Zn, Cd, and Hg. The elements, whose who have incomplete d-orbital (d1 to d9) at the time of formation of their stable ion, are called transition elements.
From the stimulus, Zn, Fe, Cu and Sc all are d-block elements because they have an electronic configuration of (n- l) d1-10 ns1-2 but, transition elements have an electronic configuration of d1 – d9 at the time of formation of stable ion.
Zn(30) → 1s2 2s2 2p6 3s2 3p6 3d10 4s2
Zn2+ (30) → … …. … … … … … 3d10
Ni (28) → … … … … … … … … 4s2
Ni2+ (28) → … … … … … … … … 3d8
Fe (26) → 1s2 2s2 2p6 3s2 3p6 3d6 4s2
Fe2+ (26) → … … … … … … … 3d6
Fc3+ (26) → … … … … … … … 3d5
Sc (21) → … … … … … … … 3d1 2s2
Sc3+ (21) → … … … … … … 3d9 2s3
It is observed from the above electronic confirmation Se3+ and Zn2+ have electronic configuration of d6 and d10 which is not satisfied with the condition of becoming transition the elements. All transition elements are either paramagnetic or ferromagnetic.
Transition elements are the elements that have incompletely filled d-orbital. On the basis of this definition, scandium and zinc do not count as transition metals – even though they are members of the d block. For example, Scandium has electronic configuration [Ar] 4s23d1 is a transition element but Zinc has electronic configuration [Ar] 4s23d10.
As zinc has totally filled d-orbital so it is not measured as a transition element. Similarly, cadmium and mercury have totally filled d-orbital. So, they are not measured as transition elements.
By contrast, copper, [Ar] 3d104s1, forms two ions. In the Cu+ ion the electronic structure is [Ar] 3d10. However, the more common Cu2+ ion has the structure [Ar] 3d9. Copper is definitely a transition metal because the Cu2+ ion has an incomplete d level. So, Sc and Zn are not transitioning elements.
D block elements may or may not form colorful complexes. Transition elements always form colorful complexes. Hence, they don’t show the variable oxidation states and can’t form a colored compound. So, all transition elements are d-block elements but all b-block elements are not transition elements.