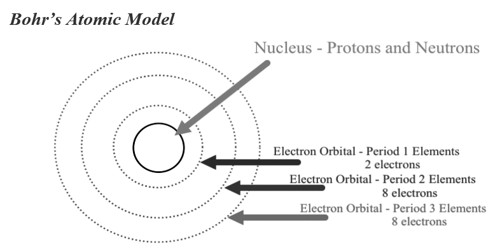
Bohr’s theory of atomic model was quite successful in explaining the stability of the atom and the line spectrum of a hydrogen atom. The Bohr atomic model theory made right predictions for lesser sized atoms like hydrogen, but poor phantom predictions are obtained when better atoms are measured. This theory also failed to explicate the Stark effect when the spectral line gets split up into fine lines in the incidence of an electric field. However, several discrepancies were observed in this model.
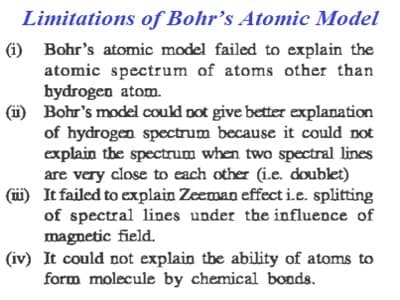
Some of the limitations of Bohr’s model are:
- Bohr’s model of an atom could not explain the line spectra of atoms containing more than one electron called multi-electron atoms. According to Bohr’s theory, one and only one spectral line can originate from an electron between any two given energy levels. But if powerful spectroscopy, are used, certain single lines are found to split into a number of very closely related lines. The existence of such a line could not be explained on the basis of Bohr’s theory.
- Bohr’s theory failed to account for the effect of magnetic field on the spectra of atoms or ions. It was observed that when the atom emitting radiations are placed in a strong magnetic field each spectral line is further split into a number of lines. This phenomenon is known as the Zeeman Effect. So, it does not explain the Zeeman Effect, when the spectral line is split into several components in the presence of a magnetic field. It was found that, when hydrogen gas was excited in a magnetic field, the produced emission spectrum was split. Bohr’s model could not account for this.
- Bohr’s theory could not explain the effect of an electric field known as the Stark effect on the spectra of atoms. If a material which gives a line emission spectrum is placed in an exterior electric field, its lines get spilled into a number of closely spaced lines. This theory cannot foretell the relative intensities of spectral lines.
- Bohr’s theory does not provide any clue to explain the shapes of the molecule arising out of the directional bonding between atoms. This model is very limited in terms of size. Poor spectral predictions are obtained when larger atoms are in question.
- The Bohr Model does not account for the fact that accelerating electrons do not emit electromagnetic radiation. According to Bohr, the radiation results when an electron jumps from one energy orbit to another energy orbit, but how this radiation occurs is not explained by Bohr.
- It is in violation of the Heisenberg Uncertainty Principle. The Bohr Model considers electrons to have both a known radius and orbit, which is impossible according to Heisenberg. Bohr assumes that an electron in an atom is positioned at a specific detachment from the nucleus and is revolving round it with explicit velocity, i.e. it is connected with a fixed value of momentum. This is against Heisenberg’s Uncertainty Principle.
The main objection of Bohr’s theory came from the new principles namely dual nature of matter and uncertainty principle. The Bohr model was a combination of quantum and traditional physics. This is a subject because it was thought that quantum physics was totally unrelated and dissimilar to customary physics. They introduced the idea of the wave character of an electron in addition to its particles character and pointed out that the path of the motion of the electron cannot be well defined. Thus these overruled the Bohr’s idea of well defined circular paths. Thus we find that Bohr’s model was only partially successful. It explained some experimental results but was unable to account for many other features of the atom. Therefore it was abandoned in the light of the modern ideas regarding the wave characteristics of matter.