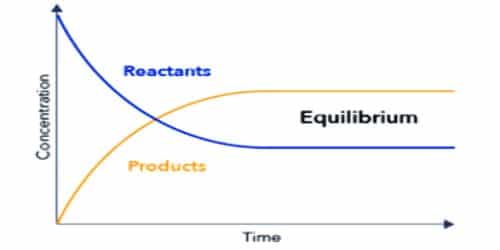
The state of chemical reaction where the rate of forwarding and the backward reaction is equal is called chemical equilibrium. In other word, chemical equilibrium is a state at which the two opposite reactions are proceeding at the same rate.
The characteristics of chemical equilibrium are:
- Reversibility:
Chemical equilibrium is only related to reversible reactions. It can only be established in the reversible reaction. Hence, it is possible to attain the state of equilibrium even in the reverse direction i.e. taking the actual products as the reactants. For example, it does not make any difference whether the reaction takes place either as –
H2 + I2 ↔ 2HI; OR, 2HI ↔ H + I2
The equilibrium state is similar in both reactions. At the point of equilibrium, all the three gases H2, I2, 2HI will be present in similar amount whether the reaction is an arrangement of hydrogen iodide or decomposition of hydrogen iodide.
- Rate:
In chemical equilibrium rate of forwarding and backward reactions will be equal. The observable properties of the system become constant at equilibrium and remain unchanged thereafter.
- The incompleteness of reaction:
If equilibrium is established then normally chemical reaction cannot proceed in any direction at equilibrium state, the amount of reactants that react to produce the product, the same amount of product is converted into reactants again. The equilibrium is obtained only in the reactions carried in a closed vessel.
- The position of equilibrium state:
The reversible reaction can be started from any order, i.e. the reaction may be started with reactants or with the product, but always the equilibrium will be established at the same position.
- Reaction area:
Chemical equilibrium is produced only in the closed system from where no substance goes out or no substances enter inside. The free energy change at equilibrium state is zero.
- Effect of factors:
State of equilibrium is maintained only as long as the reaction circumstances are maintained constant. If any system reaches an equilibrium state then that equilibrium state will continue until infinity if internal temperature, pressure, and composition remain unchanged. Modification of the surroundings such as temperature, pressure or concentration will favor the state of chemical equilibrium to change either towards the forward or backward reaction. This results in the increase of either the reactants or the products and they are not maintained at similar absorption any longer.
- Effect of catalyst:
Catalyst has no effect on chemical equilibrium. Catalyst only helps to establish equilibrium quickly. It is a substance which fastens the chemical reactions. The state of chemical equilibrium can be attained at a faster rate in the occurrence of a catalyst.
For example the reaction – 2SO2 + O2 ↔ 2SO3
Takes place at about 500 degrees and it takes a very long time to accomplish equilibrium. However, the time necessary for attaining the symmetry can be very much condensed by adding platinum. The magnitude of a catalyst is that the equilibrium can be attained at a faster rate without any changes in the composition of the reaction mixture i.e. the reactants SO2, O2, and the product SO3.
- Dynamic Character:
Chemical equilibrium is a dynamic state, not a static one. The reaction does not stop, rather it goes on. The equilibrium is dynamic in nature.
- Stability of the observable properties:
The state of chemical equilibrium is characterized by the constancy of certain properties such as concentration, density, pressure or color. However, the state of equilibrium is observed only as long as the reaction circumstances such as temperature and pressure are reserved constant.