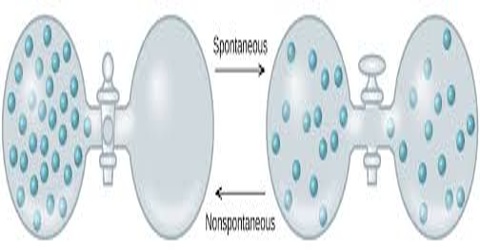
Non-spontaneous process:
Non-spontaneous processes are those that do not take place by themselves. Non-spontaneous process is a type of reaction which cannot occur without the input of work from an external source. Examples are the reverse of spontaneous processes: water does not flow from a lower level to a higher level; heat does not flow from a cold body to a hot body; solute particles in a solution do not gather together in one part of the solution etc.
Common points of Non-spontaneous process:
- Process which does not take place on its own but takes place only with the intervention of external agency or influence.
- Not natural, example; Compression of gas.
- Endothermic
- Increase enthalpy, decrease entropy.
A nonspontaneous reaction is a chemical reaction in which the standard change in free energy is positive, and energy is absorbed. Non-spontaneous processes can be carried out by supplying work or heat. For example water can be lifted from a lower level to a higher level by people or by mechanical means; in a refrigerator heat is transferred from the cold area inside it to the hot area outside it; gases can be compressed to smaller volume etc. As pointed out all these are performed by external help. Discussion of the second law of thermodynamics will lead to the understanding of the spontaneous and non-spontaneous processes.