Solution of Gas in Liquids
All gases are more or less soluble in liquids. The quantity of the gas dissolved under identical conditions of temperature, pressure and volume of the solvent, however, depends on the chemical nature of the gas and liquid. No general prediction of solubility is possible, only rough ideas can sometimes be made. Gases like He, Ne, H2, O2, N2 is only slightly soluble in water, the solubility of CO2, SO2, H2S is somewhat more, but the solubility of NH3, HCl is very high.
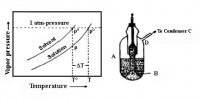
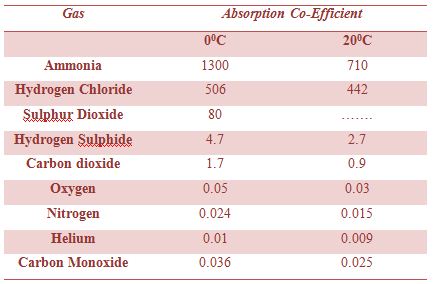
The solubility of a gas is generally defined as the volume of the gas, at the temperature and pressure of the experiment, dissolved in 1 mL of the liquid. If the volume of the gas dissolved in 1 mL of the liquid at any temperature and pressure is reduced to STP, then the volume dissolved is called absorption co-efficient. In Table, the absorption co-efficient of some common gases are given. The values refer to water as solvent and the temperatures of 0°C and 200C.
Table: Absorption co-efficient of some gases
The concentration of dissolved gas depends on the partial pressure of the gas. The partial pressure controls the number of gas molecule collisions with the surface of the solution. If the partial pressure is doubled the number of collisions with the surface will double. The increased number of collisions produces more dissolved gas.