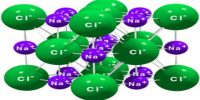
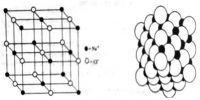
Solid Materials: Conductors, Insulators, and Semiconductors
The electrical conductivity of solids was first demonstrated by Stephen Gray in 1729. Conductivity is the ability of a material to conduct electricity. Accordingly, the solid materials can be classified as (i) conductors, (ii) semiconductors or (iii) insulators depending on their ability to conduct electricity. The electrical conductivity of semiconductors ranges from about 102 to 10-9 ohm-1 cm-1 at 25°C, while the maximum conductivity of a good conductor is about 10-4 ohm-1 cm-1 at 25°C. Substances having the conductivity of the order of about 1022 ohm-1 cm-1 at 25°C are known as insulators.
Some solids, particularly metals, are good conductors of electricity because they have a free mobile electron. These are also known as electronic conductors since electrons are the carriers of electricity. Metallic elements, their alloys, graphite, and some organic solids fall in this category. The electrical conductivity of metallic conductors decreases with rising of temperature. Semiconductors are also electronic conductors. The conductivity of semiconductors increases with temperature. Silicon, diamond and gallium arsenide are examples of semiconductors. Semiconductors have generally very low conductivity, but their conductivities may be significantly enhanced by incorporating impurities into the structure. Materials with very low electrical conductivities are known as an insulator. Glass, wood etc. are insulators. There is another class of solids known as superconductors. They have almost zero resistance to the flow of electricity. Certain metals and alloys exhibit superconductivity when cooled to sufficiently low temperatures. It was discovered in 1911 by Kamerlingh Onnes. Superconductors find applications in superconducting magnets, energy technology, telecommunications, computing, and medicine.
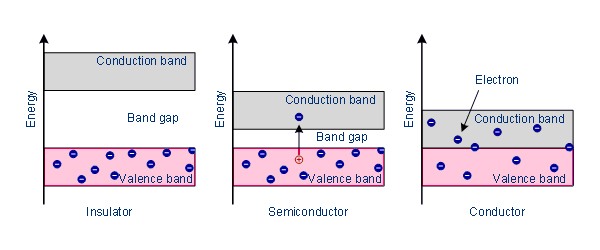
Fig: Band Model
The conducting and insulating properties of solids can be explained by considering the ‘Band Theory’ of metals. According to this theory, in a metallic crystal, the atoms are closely packed together in such a way that the energy levels of electrons of each atom are affected by the energy levels of electrons of the immediate neighbors. As a result, the atomic orbitals of neighboring atoms overlap with each other. According to the molecular orbital theory, the overlapping of atomic orbitals leads to the formation of bonding and antibonding molecular orbitals. Since the number of atoms even in a small piece of metal is very large, the number of molecular orbitals thus formed must also be very large. These molecular orbitals are so closely spaced on the energy scale that they are described as ‘Bands’. The highest filled energy band is called ‘valence band’ and the next higher one is called the ‘conduction band’ (Figure). The conduction hand is separated from the valence band by an energy gap, also known as the forbidden band. The Fermi level lies between the valence band and the conduction band.
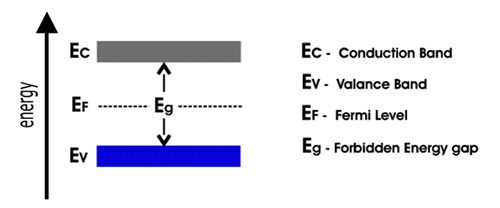
Figure: Schematic diagram of the conduction band, valence band, and Fermi level
The important energy hands are (a) the topmost valence bands, i.e., the energy levels of the valence electrons, and (b) the conduction band which is at a higher energy level than the valence bands.