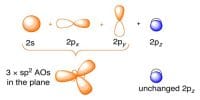
In any period as one goes from left to right the atomic number of elements increases. With the increase of atomic number, the number of electrons increases but there is no increase of electron orbit. As atomic number increases, the number of proton in the nucleous also increases. Due to the higher positive charge on the nucleous attracts the electrons more tightly. As result the atomic radius decrease.
Hence the ionic radius also decreases due to the increase of positive charge on the cations. Thus the size of ions gradually decreases in a period from left to right.
The order of ionic size of elements of 3rd period is Na+ > Mg2+ > Al3+ > Si4+