Saturated and Unsaturated Solubility
A Saturated solution is a solution that has dissolved the maximum amount of solute. An Unsaturated solution describes a solution that has a less than the max amount of solute dissolved. A saturated solution is when the solute can dissolve in the solvent. For example, if you have a bottle of water and you pour lemonade crystals into the water, and it dissolves, the solution is saturated.
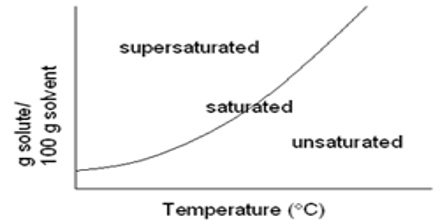
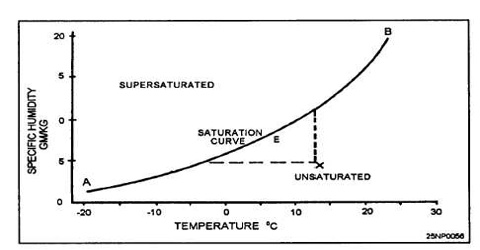
At equilibrium no more solid appears to dissolve. For example, if we add 45.0 g of KCl to 100 g of water at 300C only 37.0 g of the solute will be found to dissolve and the remaining 8.0 g will remain at the bottom of the solution in the solid undissolved state. A solution that contains as much of the dissolved solid as it can hold at a particular temperature is said to be saturated. In other words a solution that is in equilibrium with solid solute is a saturated solution. If it contains less than that required for saturation the solution is said to be unsaturated, the solubility of a solid in a liquid is usually defined – “as the amount of the solid in grams which is dissolved by 100 g of the liquid to make a saturated solution at a definite temperature”. Thus the solubility of KCl in water at 30°C is 37.0 g means that at 30°C temperature 100 g of water can dissolve a maximum of 37.0 g of the salt. Since in a saturated solution dynamic equilibrium exists, the solubility of the solute in 1000 g of the solution can be considered as equilibrium constant, KC.
The terms saturated and unsaturated are in no way directly related to the terms concentrated and dilute. A saturated solution of silver chloride at room temperature contains only 0.000089 g of AgCl per 100 g of water. This is a very dilute solution as it contains very small amount of the solute in 100 g of water. On the other hand a saturated solution of LiClO3 in 100 g of water at the same temperature contains 500 g of the solute. A solution of LiClO3 in 100 g water containing 300 g of the solute is unsaturated but definitely concentrated. Thus a saturated solution can be dilute and an unsaturated solution can be concentrated.