Rutherford’s Nuclear Model of Atom: Rutherford and his students (Hans Geiger and Ernest Marsden) bombarded very thin gold foil with α-particles. Rutherford’s famous α-particle scattering experiment is represented in Figure. A stream of high energy a-particles from a radioactive source was directed at a thin foil (thickness – 100 nm) of gold metal.
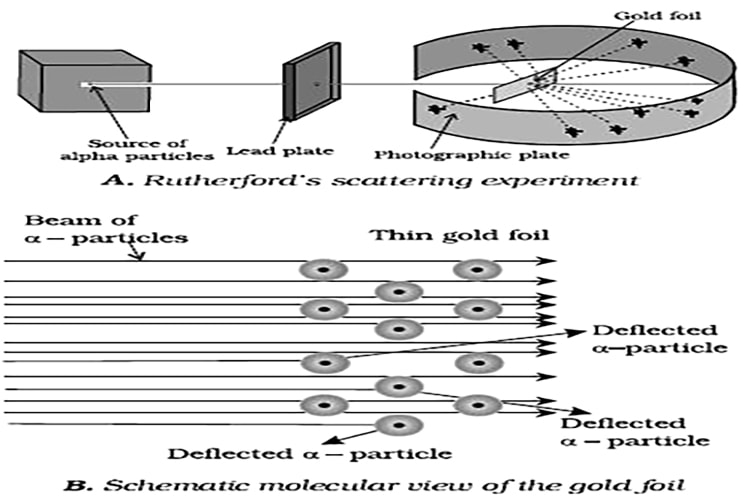
The thin gold foil had a circular fluorescent zinc sulphide screen around it. Whenever a-particles struck the screen, a tiny flash of light was produced at that point.
The results of scattering experiment were quite unexpected. According to Thomson model of atom, the mass of each gold atom in the foil should have been spread evenly over the entire atom, and α- particles had enough energy to pass directly through such a uniform distribution of mass. It was expected that the particles would slow down and change directions only by a small angles as they passed through the foil. It was observed that:
(1) most of the α- particles passed through the gold foil un-deflected.
(ii) a small fraction of the α-particles was deflected by small angles.
(iii) a very few α- particles (≈1 in 20.000) bounced back, that is, were deflected by nearly 180. On the basis of the observations.
Rutherford drew the following conclusions regarding the structure of atom:
(i) Most of the space in the atom is empty as most of the α-particles passed through the foil un-deflected.
(ii) A few positively charged a- particles were deflected. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom as Thomson had presumed. The positive charge has to be concentrated in a very small volume that repelled and deflected the positively charged α- particles.
(iii) Calculations by Rutherford showed that the volume occupied by the nucleus is negligibly small as compared to the total volume of the atom. The radius of the atom is about 10-10 m. while that of nucleus is 10-15 m.









