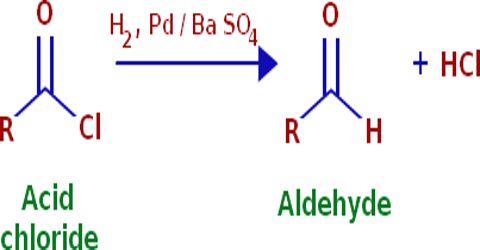
Rosenmund’s Reaction:
When acetyl chloride is reduced with hydrogen in the presence of ‘poisoned’ palladium catalyst, it forms aldehydes. This reaction is called Rosenmund’s reaction.
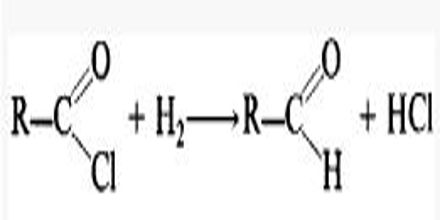
Barium sulfate (BaSO4) is used as poisoned acid.
Catalytic hydrogenation of an acid chloride to form an aldehyde; the reaction is in the presence of sulfur to prevent the subsequent hydrogenation of the aldehyde.
The synthesis of aldehydes from acyl halides can be carried out in two ways. The first method employs catalytic hydrogenation with a metallic catalyst, for example platinum. This reaction is referred to as Rosenmund reduction. Alternatively, acyl halides can be reduced to aldehydes with complex metal hydrides. Mild reducing agents, such as sodium borohydride and lithium tri-tert-butoxyaluminum hydride, have to be used to stop the reaction at the aldehyde stage.