Relaxation Effect of Electrolytic Solution
When an electrical potential is applied to an electrolytic solution, two effects produced by the ionic atmosphere prevent the ions to move at the expected speed and hence conducting the amount of current. One of these effects is Relaxation effect.
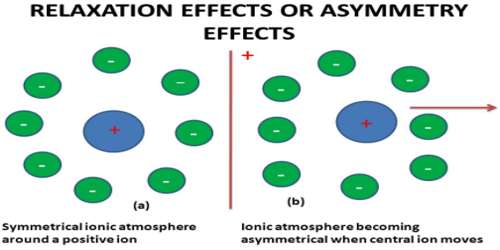
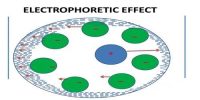
Relaxation effect (sometimes known asymmetry effect)
In an electrolytic solution, each ion is surrounded by an atmosphere of counter-ions, e.g., ions having opposite charge (ionic atmosphere). In other words, cations are surrounded by an atmosphere of anions and vice versa.
The effect of the relaxation of the ionic atmosphere on the rate of diffusion‐controlled reaction at short times was investigated. In the absence of an external field the ion, along with its surrounding ionic atmosphere, remains fairly steady, and the ionic atmosphere remain symmetrical around the central ion. When such a system is subjected to an external electric field, the central ion and its ionic atmosphere moves toward the oppositely charged electrode. As the central ion starts moving out of the ionic atmosphere a new ionic atmosphere of opposite charge starts building around it. The old ionic atmosphere will eventually die out. The destruction of an ionic atmosphere and the completion of the formation of a new ionic atmosphere do not take place all at once. There is a time lag between these two processes. This time lag is termed as relaxation time. During this relaxation time the old ionic atmosphere pulls the moving ion backward and thus retards its motion.
The generalized diffusion equation, in which the time‐dependent friction is taken into account, was solved numerically together with the radiation boundary condition to predict the short‐time behavior of the rate constant of an encounter reaction between a neutral target molecule at rest and migrating ions.