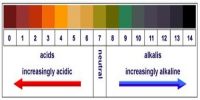
Acids are a family of chemicals which react in a similar way. For example, acids found in foodstuff give a characteristic sharp taste.
Metal hydroxides, oxides or carbonates are part of another family called base. A soluble base is called an alkali.
Acids often react to produce ionic compounds referred to as salts. Note that salt is a chemical term that covers any ionic metal compounds, e.g. potassium hydroxide, calcium carbonate, etc.
The salt formed will depend on the metal cation of the base and the non-metal or polyatomic anion of the acid.
Typical reactions of acids
i. Acid + Metal → Salt + Hydrogen
e.g. 2HC1 (aq) + Mg (s) MgCl2 (aq) + H2 (g)
ii. Acid + Base → Salt + Water
e.g. H2 SO4 (aq) + 2NaOH (aq) → Na2 SO4 (aq) + 2H2O (I)
iii. Acid + Carbonate → Salt + CO2 + Water
e.g. 2HNO3 (aq) + CaCO3 (aq) → Ca(NO3)2 (aq) + CO2 (g)+ H2O (1)