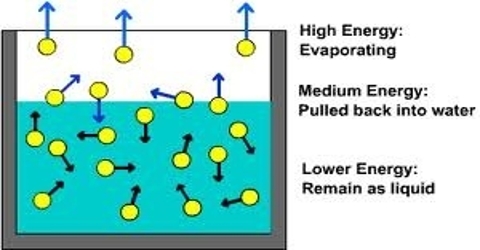
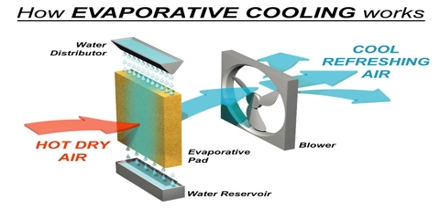
Cooling by rapid evaporation of volatile liquids depends on the fact that when the liquid evaporates it takes up its latent heat of evaporation from the liquid itself. A familiar example is the cold felt when a few drops of ether or acetone is taken in the hand and allowed to evaporate.
Pictet (1877) was able to liquefy oxygen by using this principle. By evaporation of liquid sulfur dioxide under reduced pressure around carbon dioxide, the latter was liquefied in large quantity. When liquid carbon dioxide was alloyed to evaporate under reduced pressure, a temperature of about -130°C was obtained and this temperature was sufficient to bring about liquefaction of compressed oxygen. This process of attaining low temperatures and liquefying gases by successive evaporation (under reduced pressure) of different liquids, using liquid A to cool the gas B and so on, is known as the cascade method.
The method was used by Keesom to attain a temperature of 0.71°K by boiling liquid helium under reduced pressure, but helium could not be liquefied by the use of this method alone.