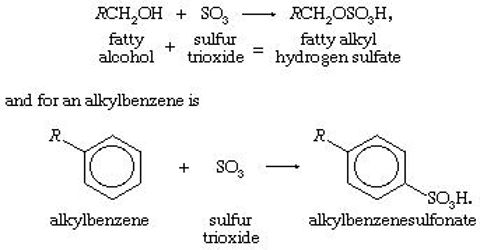
Detergent: Detergent or synthetic clearing agent is the sodium salt of long chained alkyl/aryl hydrogen sulphate or sulphonate or alkylated fenoxy/ ammonium compounds. The general formula of detergent is R/Ar – SO4 /SO3 – Na, (R = 12 – 18C).
Detergent production are of three types:
Anaionic detergent: Its anion part acts as detergent. Example:
- Sodium lorayl sulphate [CH3(CH3)10CH2 – O – SO2 – O–Na+]. It is produced from loryl alcohol (C12H25OH) which is derived from palm oil or coconut oil.
- Sodium alkyl benzyl sulphonate or ABS detergent.
Cataionic detergent: Its cation part acts as detergent. Example:
- Tertiary amine bromide. C15H31 – CH2 – N+ (CH3)2 Br–
- Tertiary aniline chloride.
Non-ionic detergent: These are charge less alcoxy poly ethelene Oxide and alkylated phenoxy polyethelene oxide. Washing powder is produced by mixing anionic detergent with ion-ionic ones. They don’t product any froth & aren’t attacked by acid or alkali. That’s why; they are used in washing machine. Tri-poly phosphate is used as in powerful washing soda to control PH.
General composition of Commercial detergent: 20-40% detergents + different types of builder, filler, dyes & other compounds.