Preparation of Polyesters
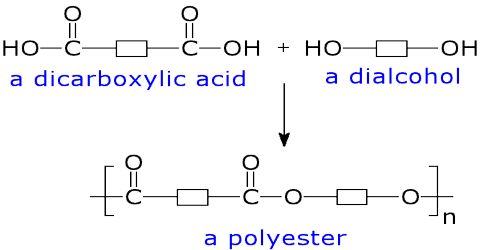
They are polymers those can be produced by polymerization of esters formed due to condensation reaction between di-carboxyllic acid & di-hydric alcohol. A polyester is a polymer (a chain of repeating units) where the individual units are held together by ester linkages.
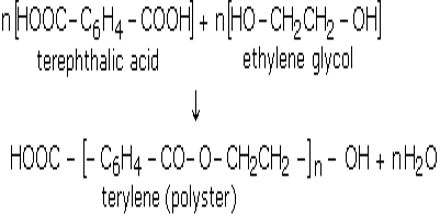
Production of Terylen: Ethan -1, 2 -diol and 1, 4 -benzene dioic acid react to form polyester called terylen. It is known as “Dacron” in the U.S.A. Each chain of terelyn contains 80 ester units. Polyesters are prepared by the condensation polymerization of a dicarboxylic acid with a dihydric alcohol with the elimination of water. For example, terylene (or Dacron) is prepared from terephthalic acid and ethylene glycol by condensation polymerization with the elimination of water.
A polyester is made by a reaction involving an acid with two -COOH groups, and an alcohol with two -OH groups.
In the common polyester drawn above:
- The acid is benzene-1,4-dicarboxylic acid (old name: terephthalic acid).
- The alcohol is ethane-1,2-diol (old name: ethylene glycol).
Use Production of natural cotton by mixing dacron with carpus (in 60:40 or 80:20 ratio), Terylen is also used in the production of shirts, pants, socks etc.