Preparation of Crystals
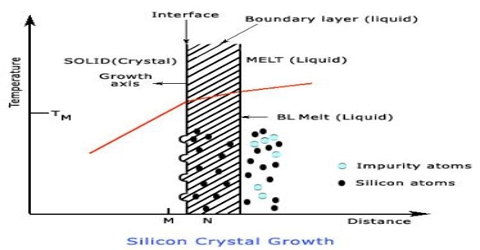
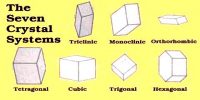
Crystals of solid substances are formed when saturated solutions of solids are cooled slowly. The process is called crystallization. The size and purity of crystals depend to a large extent on the rate at which they are formed. Formation of the crystals is accompanied by evolution of a large amount of heat (heat of fusion). The slower the rate of formation the purer and larger is the crystal, because the atoms and molecules have time to find their proper positions in the crystal lattice. Crystals of different substances and of varying sizes have been prepared by a good number of ingenious methods. Commercial preparations of most substances are almost always highly unsuitable for microscopic examination because crystal quality is seldom of interest for manufactured chemicals. There are several techniques that can be used to make crystals suitable for microscope examination that require virtually no special equipment. Some of the commonly used methods are:
(a) Ceramic method,
(b) Microwave synthesis,
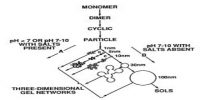
(c) Sol-Gel method.
(d) Precursor method,
(e) Hydrothermal method,
(f) Chemical Vapour Depositions (CVD) method.