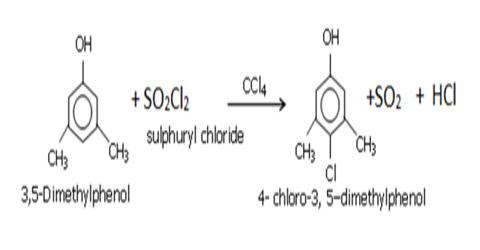
Preparation of Carbon Tetra Chloride or Pyrene
Preparation:
Commercially, Carbon Tetra Chloride is prepared by chlorination of chlorine. Carbon tetra chloride can be prepared from phosgene at 300–450°C. Carbon Tetra Chloride is prepared by the action of chlorine on carbon disulphide in the presence of iodine, which acts as a Catalyst. Carbon tetra chloride may also be prepared by the free radical substitution of the hydrogen atoms of methane by chlorine. Carbon tetra chloride may also be prepared by the free radical substitution of the hydrogen atoms of methane by chlorine.
CH4 + 4Cl2 →light→ CCl4 + 4HCl
It can also be produced from CS2 & Cl2 in the presence of dehydrated AlCl3.
CS2 + 3Cl2 → dehydrated AlCl3→ CCl4 + S2Cl2
The boiling point of CCl4 is 770C.
Uses:
- It is a good solvent for oils, fats, rubber, wax etc.
- Also used in dry-washing of silk, washing of sanitary tiles & different machineries.
- For eradication of hook worm & insects.
- For production of freons.