Standard reduction potential E° values can be used to predict whether a redox reaction will happen spontaneously or not.
Find the standard reduction potential of the two half reactions:
- The more positive standard reduction potential will occur as a reduction.
- The more negative one will occur as an oxidation.
A question one can ask is can silver reduce Cu2+ ?
Ag+ (aq) / Ag (s) E° = +0.80 V
Cu2+ (aq) / Cu (s) E° = +0.34 V
By comparing the two E° values it is possible to understand how the reaction is likely to proceed. + 0.80 is greater than + 0.34, so Ag+ (aq) → Ag (s) must be the reduction reaction. Then the reaction between Ag and Cu2+ (the opposite of this) will not take place spontaneously.
Imagine how the possible reaction could be part of an electrochemical cell. As voltaic cells always have a positive cell emf, calculating Ecell can tell whether a possible redox reaction will proceed spontaneously or not.
Note that although calculating the einf of a possible reaction will tell whether a reaction will happen, it does not give information on how quickly such a reaction would happen.
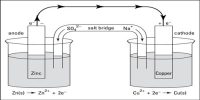
For example, can silver reduce Cu2+ ? This reaction would involve a cell where silver was oxidised at the anode and copper ions were reduced at the cathode as follows:
Ag (s)│Ag+ (aq)││Cu2+ (aq)│Cu (s)
Remember that:
Ecell = Eocathode – E0anode
So Ecell = + 0.34 — (+0.80) = — 0.46 V. As the calculated value is negative, the reaction will not take place spontaneously.
Similarly, would there be a reaction between Na and Mg2+?
Ecell = (-2.37) – (-2.71) = +0.34 V.
Ecell is positive therefore, the reaction would proceed.
These ideas are expressed more simply by the concept of an activity series of metals. The most reactive metals are those which are most easily oxidized. As the more positive E° values indicate species which are the strongest reducing agents, the most reactive metals are those which have the most negative metal ion/metal E° value.