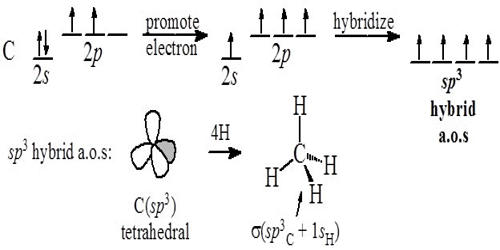
Hybridization is the process of the formation of new equivalent orbitals. It is the concept of intermixing of the orbitals of an atom having nearly the same energy to give exactly equivalent orbitals with the same energy, identical shapes, and symmetrical orientations in space.
The orbitals participating in hybridization should have nearly the same energy. Thus in the formation of methane, the 2s and 2p orbitals of carbon have nearly the same energies, so that the recasting of orbitals is possible.
Conditions of Hybridization
(i) Only the orbitals present in the valency shell get hybridized. Atomic orbitals undergoing hybridization should belong to the same atom or ion. Only the orbitals present in the valence shell of the atom are hybridized.
(ii) The orbitals taking part in hybridization must have only a small difference in enthalpies. Atomic orbitals participating in hybridization should have nearly the same energy. Thus 2s and 2p can hybridize, 3s and 3p can also hybridize, but 2s and 3p cannot.
(iii) Both filled and half-filled orbitals get hybridized. The total number of hybrid orbitals formed is equal to the number of atomic orbitals involved in the hybridization process.
(iv) The orbitals undergoing hybridization generally belong to the valence of the atom. Only those orbitals which have approx. equal energies and belong to the same atom or ion can undergo hybridization.
(v) The energy difference between orbitals undergoing hybridization should be small. All hybrid orbitals are identical with respect to energy and directional character.
(vi) It can take place between completely filled, half-filled, or empty orbitals. The hybrid orbitals may differ from one other in their orientations. The shape of the hybrid orbitals is different from that of the original atomic orbital.
(vii) The promotion of electrons is not a must before hybridization takes place. Similar to atomic orbitals, each hybrid orbital can have a maximum of two electrons.
(viii) All the orbitals of the valence shell may or may not take part in hybridization. It is not necessary whether half-filled or fully filled orbital can participate in hybridization.
(ix) Hybridization takes place at the time of bond formation only. The hybrid orbitals have maximum symmetry and definite orientation in space so that the mutual force of repulsion of electrons is avoided.
(x) The hybrid orbitals are concentrated in one particular direction to achieve greater overlapping. There are one large lobe and one small lobe representing overlapping and non – overlapping regions.