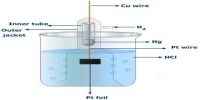
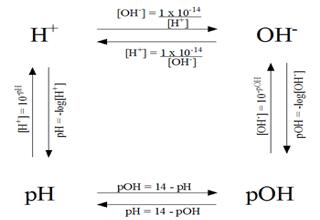
A measurement of the hydroxide ion concentration, similar to pH, is called pOH.
The pOH of a solution is defined as the negative logarithm of the molar hydroxide- ion concentration as follows:
pOH = -log10 [OH-]
Because KW = [H+] x [OH–] = 1.0 x 10-14 at 25°C, one can demonstrate that
pH + pOH = 14.00
Example
A solution has a hydroxide-ion concentration of 1.9 x 10-3 M. What is the pH of the solution?
Firstly, calculate the pOH:
pOH = -log10 (1.9 x 10-3) = 2.72
Then, calculate the pH:
pH = 14.00 — 2.72 = 11.28