Physical Properties of Haloalkanes:
Haloalkanes are hydrocarbons in which hydrogen in normal alkane is replaced by a halogen. They are slightly soluble in water. Pure haloalkanes are colourless.
(i) Physical state and odour: The lower members e. g. CH3F, CH3Cl, CH3Br, H3C—CH2F etc. are gases. CH3-I, CHCl3 and the majority of the higher members upto C18 are sweet-smelling liquids, but the higher homologoues are odourless solids. Alkyl halides are sweet-smelling; but they are toxic. So they should be handled cautiously.
(ii) Solubility: Haloalkanes are slightly soluble in water, but they are readily soluble in organic solvents like alcohol, ether etc. readily.
(iii) Boiling points: Haloalkanes have higher molecular masses than the corresponding parent alkanes. The melting and boiling point of haloalkane increases in the order:
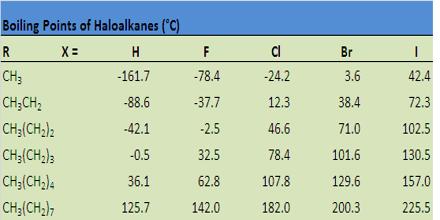
For isomeric halokanes, the boiling points decrease with branching. Thus, a branched chain isomer will have lower boiling point than the straight chain isomer.
Due to having polarity in C-F & C-CI bonds, the B.P of fluoro-alkane & chloro-alkane is higher than that of the corresponding parent alkanes. But bromo & iodo-alkanes have lower B.P. than that of corresponding parent alkanes due to the decreased volume of bromo & iodo-alkanes, in comparison to that of parent alkanes.
(iv) Specific gravity: Iodides & bromides are heavier than H2O; but fluoride & chloride are lighter. Specific gravity decreases with the increases of molecular mass.
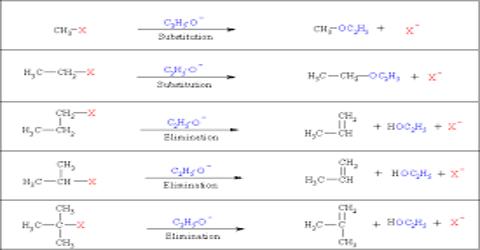
Haloalkanes give following reactions
- Nucleophilic substitution reaction (SN reaction)
- Elimination reaction
- Reduction reaction
- Reaction with metals to form organo-metalic compounds.