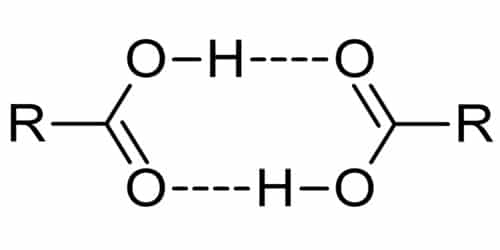
Carboxylic acids exhibit strong hydrogen bonding between molecules.
Physical properties of carboxylic acids
- Physical state:
The first three members (up to 3 carbon atoms) are colorless, pungent-smelling liquids. The next six members are oily liquids having an unpleasant smell. The higher members are colorless waxy solids. Many carboxylic acids are colorless liquids with unpleasant odors. They also participate in hydrogen bonding due to the presence of the carbonyl group (C=O) and the hydroxyl group.
- Solubility:
Carboxylic acids are soluble in water. The acids are polar. The lower members of the aliphatic carboxylic acid family (up to C-4) are highly soluble in water. They do not dimers in water but forms hydrogen bonds with water. The solubility decreases with the increase in the size of the alkyl group. All carboxylic acids are soluble in alcohol, ether, and benzene, etc. They are polar and due to the presence of the hydroxyl in the carboxyl group, they are able to form hydrogen bonds with water molecules. Benzoic acid (the simplest aromatic carboxylic acid) is sparingly soluble in cold water, and the solubility increases with increases in temperature. Benzoic acid is soluble in alcohol, ether, etc.
- Odor
Carboxylic acids tend to have strong odors, particularly those that are volatile. They generally have a strong sour smell. Common odors can be found in vinegar, which contains ethanoic acid, and rancid butter, which contains butanoic acid. Esters of carboxylic acids tend to have pleasant odors, so they are generally used to make perfumes. However, their esters have pleasant odors and are therefore used in perfumes.
- Acidity
Carboxylic acids are weak acids. They partly dissociate into H+ cations and RCOO– anions in neutral aqueous solvents such as water. e.g. at RTP, only 0.4% of ethanoic acid molecules dissociate in water. The solubility of compounds containing the carboxyl functional group in water depends on the size of the compound.
- Boiling Point:
The boiling points of the carboxylic acids increase with molar mass, i.e. an acid-containing larger number of C atoms has a higher boiling point. The boiling point of a carboxylic acid is generally higher than that of water.
Carboxylic acids have a higher boiling point than alcohols of comparable molecular mass.