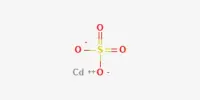
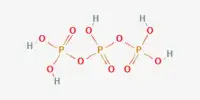
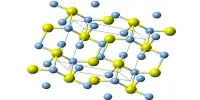
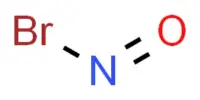P is more reactive than N
Though, N is less reactive at room temperature, P is much reactive, especially white phosphorus is very reactive. The enthalpy of P – P bond is 200 kJ/mol bond. N ≡ N triple bond is much stronger and that’s why the enthalpy is so high. So, to break down P-P bond is much easier than to break N ≡ N bond. Again P4 molecule has a tetrahedral shape and the bond angle is 60°. While N2 molecule is linear and the bond angle is 180°. So, more stress is needed to break N ≡ N bond than P-P bond. So P is much reactive than N.