Osmosis Pressure
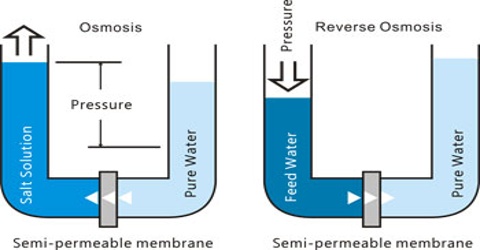
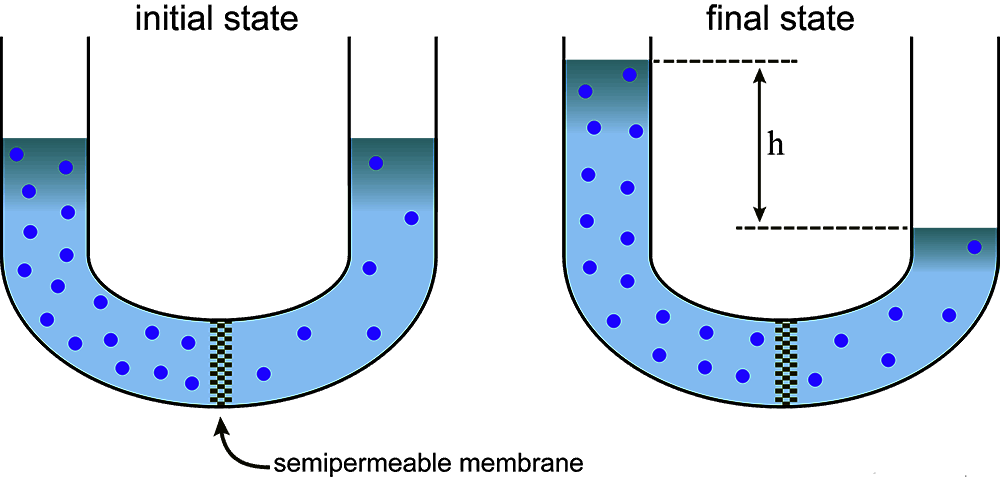
We know that a gas or vapour spreads out from a region of high pressure to a region of low pressure. Similarly, if a concentrated solution and a dilute solution of the same solute are placed together in the same container the solute particles move from the concentrated solution into the dilute solution. These spreading out of gases or solutes in solution is spontaneous and continue fill the mixtures are homogeneous. The process is known as diffusion. This migration of the gas molecules or solute particles is caused by translational motion of the molecules/particles. If, however, solution is separated from the solvent (or a dilute solution is separated from a concentrated solution) by a semi-permeable membrane a reverse process will take place in which only the solvent molecules migrate through the membrane but not the solute molecules. This is shown in figure.
Osmosis Pressure
Due to difference in concentration the solvent molecules well go on migrating through the semi-permeable membrane unless it is presented by external forces to do so. This phenomenon of selective migration of the solvent, molecules through the separating semi-permeable membrane to the solution is called osmosis. As a result of osmosis the solute concentration on either side of the Membrane become equal at equilibrium. Due to spontaneous inflow of the solvent through the membrane to the solution side, a pressure is developed in the solution side. This continues to increase as long as the solvent flows. However, the inflow of the solvent can be stopped from the beginning by application of an appropriate external pressure to the solution side. For equilibrium the external pressure must be equal to the pressure developed by osmosis. This external pressure is denoted by P.