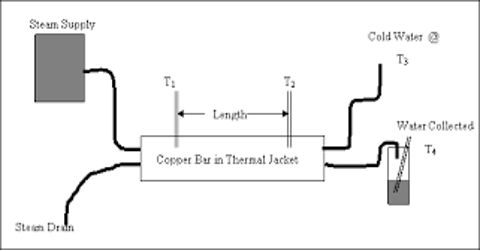
Observe the Thermal Conductivity of Metal (Copper)
Required Accessories: Thick copper wire (20 cm), two pieces of cork, safety matches, candle or spirit lamp.
Procedure: Insert the copper wire carefully through the cork so that the cork remains in the middle of the wire. Lit the candle. Now, holding one end of the wire, place its other end on the candle-flame. In this way hold the wire until your hand does not feel warm (hot).
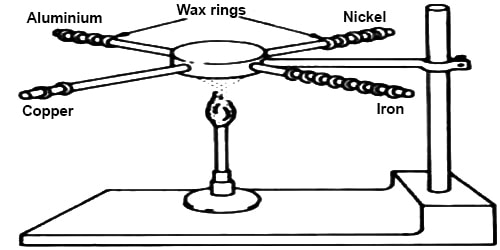
Here what is the reason of using cork? It is used so that the heat from the flame could not reach directly to the hand. Why do you feel the heat at the end of the copper wire that you are holding in your hand? One end of the wire receives the heat from the flame and it travels to other end because copper is a good conductor. If it was not so, you may not have felt the heat. Therefore, it is proved by this experiment that copper is a good thermal conductor. In fact, all metals conduct heat like copper. That is why metals are used where thermal conduction is essential. (For example: in refrigerator, air conditioner. solar panel etc.). Therefore, it is our moral duty to ensure proper uses as well as to avoid wastage of metals.