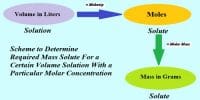
Molar solution is a standard solution
The solution whose strength is known accurately is called standard solution. Again, molar solution is that solution when one mole of a solute remains in one litre solution that means the strength of molar solution is known. So, molar solution is called a standard solution.
E.g.: 1M NaOH solution means that the strength of NaOH is 1M which is known. So, 1M NaOH solution is standard solution.