Liquefaction of gases is the procedure by which a gas is transformed into a liquid. For example, oxygen usually occurs as a gas. However, by applying enough amounts of pressure and by tumbling the temperature by an adequate amount, oxygen can be changed to a liquid.
The main two processes of liquefaction of gases are-
- By using Freezing Mixture
- By Joule Thomson Effect
- Claude’s method.
In general, gases can be liquefied by one of three general methods: (1) by compressing the gas at temperatures less than its decisive temperature; (2) by making the gas do a few kinds of work alongside an exterior force, causing the gas to drop energy and alter to the liquid state; and (3) by making gas does work alongside its own interior forces, also causing it to drop energy and turn to liquid.
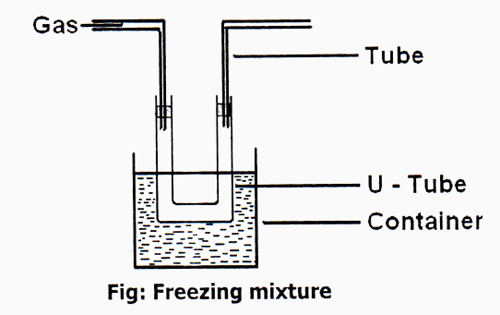
1) By using Freezing Mixture: Temperature of a gas cannot be lowered very much by using a Freezing mixture. For example, ammonia has a decisive temperature of 406K (271.4°F [133°C]). This temperature is fine above room temperature, so it is comparatively easy to renovate ammonia gas to the liquid condition plainly by applying enough pressure. Therefore, only a few gases like NH3, CO2, Cl2, etc could be liquefied by process temperature can be decreased to-20°C by using mixture of common salt and ice, instead of common salt using mixture of CaCl2 and ice temperature can be decreased to -54°C and temperature can be lowered into -110°C by using mixture of solid CO2 and Ether. At its decisive temperature, that pressure is 112.5 atmospheres, even though the cooler the gas is to start on with, the less pressure is required to formulate it compress.
(2) By Joule Thomson Effect: When a highly compressed gas is allowed to expand through a porous plug into a region of low pressure so that no work is done against the external pressure, the temperature of gas falls down. This is called Joule Thomson effect of an ideal gas.
During the state maximum intermolecular attraction force is occurred. During expansion the molecules get far away from each other, as a result, they need to work against the intermolecular attraction force. The energy for this purpose is taken from the gas itself, so its temperature falls.
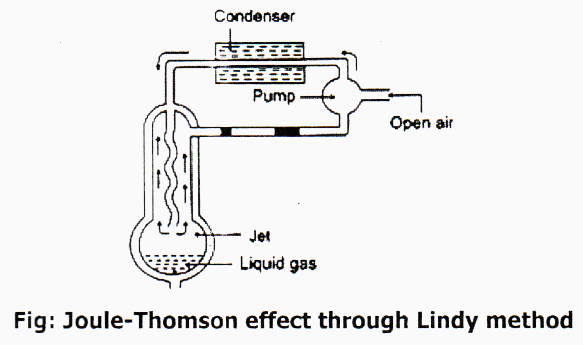
German Scientist Lindy invented the commercial process of liquefaction of gas taking Joule- Thomson effect as principal. This procedure is based upon Joule-Thomson effect which states that “When gas is allowed to expand adiabatically from a region of high pressure to a region of enormously low pressure, it is accompanied by cooling.” According to the process of scientist Lindy, by compression and expansion of gas repeatedly it temperature decreases gradually and reach critical temperature, then the gas turns into liquid. This process is at present used commercially to liquefy air.
(3) Claude’s method: This procedure is based upon the principle that when a gas expands adiabatically against an exterior pressure (as a piston in an engine), it does a few peripheral works. Since work is done by the molecules at the cost of their kinetic energy, the temperature of the gas falls causing cooling.