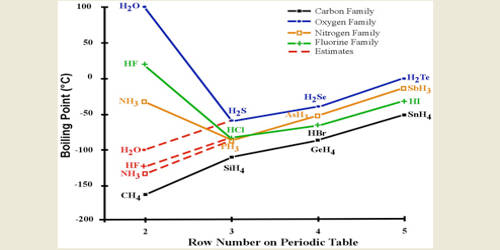
Melting and Boiling points of Hydrogen Fluoride (HF) are higher than Hydrochloric acid (HCl), Hydrobromic acid (HBr) and Hydrogen iodide (HI).
Fluorine has the highest electronegativity of an atom. When fluorine bonds with hydrogen, the polarity is so strong that it begins to exhibit the property of hydrogen bonding, which is in concentrate just an excessive dipole. Chlorine simply doesn’t have the pull of its halogen counterpart and can’t form that polar of a molecule.
According to Fajan’s rule with the increase of the size of anions, the tendency to be polarized increases. Because, as the size of anion increases, the distance between its nucleus and outermost electrons increases and hence the nucleus can attract the electrons less. So the cation can more easily deform the anion. Therefore in any group in the periodic table the tendency of the anion to be polarized increases from top to bottom. For example, the radii of the halide ions are as follows.
F– (1.33A) < Cl– (1.81A) < Br (1.96A) < I (2.20A) and the tendency to be polarized increases in this order.
This is clearly demonstrated by the gradual decrease of melting and boiling points of Hydrogen halides Hf > HCl > HBr > HI
Again, due to the presence of hydrogen bonds in Hydrogen Fluoride (HF) molecule, it’s melting and boiling points are higher. Fluorine has a higher electronegativity than the other halogens which means for fluorine it undergoes hydrogen bonding which gives it a boiling point of about 19 degrees Celcius. Whereas hydrogen chloride boils at -80.05 degrees Celcius. The other halogens being less electronegative than Fluorine would therefore not form hydrogen bonds to a degree that fluorine does.
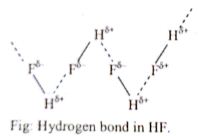
Hence the intermolecular force between the molecules of HF compound increases which causes higher melting and boiling points. But in the case of HCl, HBr, and HI, they can’t form a hydrogen bond. Hence their melting and boiling points are lower than HF.
Due to the high electronegativity of fluorine hydrogen bonds can be formed between HF molecules. Hydrogen bonds require more energy to break that London Forces. The other halogens are not as electronegative and so other hydrogen halides cannot form hydrogen bonds between molecules. Therefore more energy is required to break the intermolecular forces in HF than the other hydrogen halides and so it has a higher boiling point. Stronger hydrogen bonding leads to a higher boiling point. Therefore you have a higher charge difference between the hydrogen atom and the fluoride atom leading to a greater attraction and strongness of the hydrogen bonding.