Measurement of Vapour pressure procedure devised by Smith and Menzies
Smith and Menzies devised a very simple apparatus for measuring vapour pressures of liquids. It consists of a bulb-tube tab a narrow and bent outlet, which is tied to the bulb of a thermometer as shown in Figure. The bulb is partly filled with the liquid under investigation. The thermometer bulb is then introduced into a boiling tube containing a liquid of high boiling point, e.g., paraffin; the boding tube is connected to pressure reservoir, pressure gauge and a vacuum system as in Figure.
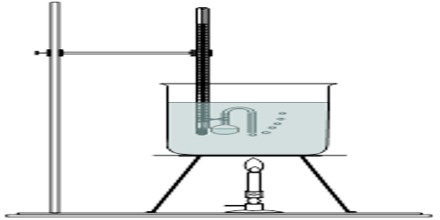
Fig: Apparatus of Smith and Menzies
The paraffin in the boiling tube is brought to convenient temperature in a thermostat and the tube is evacuated. After some time the liquid in the bulbs starts boiling as is indicated by the escape of a stream of bubbles. Air is allowed to enter the system very slowly through D (Figure) until the bubbles just cease to come out of the tube. The temperature and pressure are recorded. The pressure is corrected for the pressure of the column of paraffin above the bulb-tube. The corrected pressure reading is the vapour pleasure of the liquid at this temperature. In a similar way vapour pressures at other temperatures may be measured.
The gas saturation or transpiration method is more suitable for determining lowering of vapour pressure in dealing with dilute solutions but may be adapted for measurement of vapour pressure of pure liquids.