Measurement of Vapour pressure procedure devised by Ramsay and Young
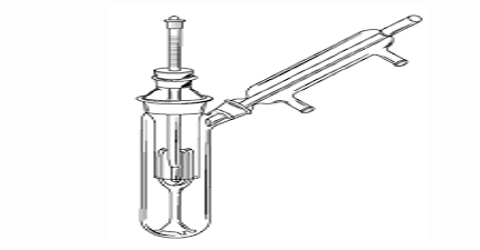
In the procedure devised by Ramsay and Young the external pressure is fixed and the temperature at which the liquid boils is then measured. The external pressure is equal to the vapour pressure of the liquid as this temperature. The apparatus is shown in Figure.
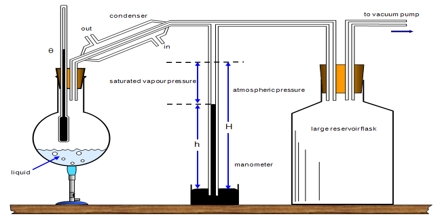
Figure: Ramsay and Young Apparatus
It consists of a boding tube fitted with a rubber stopper through which passes a thermometer. The bulb of the thermometer is covered with the absorbent cotton which is kept moist with the liquid to he studied. This it achieved by dropping a slow stream of the liquid from a dropping funnel as shown in figure. The boiling tube is provided with a side tube which is connected to a vacuum system as in Figure. The liquid film in contact with thermometer bulb is warmed by placing the boiling tube in a liquid bath, the temperature of which is maintained 100-20°C above that indicated by the thermometer.
For making a measurement the pressure is maintained at a given low value by adjusting the air inlet in the vacuum system. The temperature of the tube and its contents are then raised until the thermometer reading does not change when a drop of liquid is allowed to flow into the cotton. The reading of the thermometer and the pressure reading are taken. At this temperature the liquid is in equilibrium with the vapour. Hence the pressure recorded is the vapour pressure of the liquid at this temperature.