Liquid State
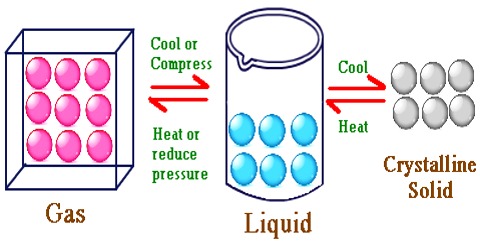
Liquids are characterized by very till compressibility. Unlike solids, liquids have no definite shape. Liquids, however, have definite volumes under given conditions.
They have high densities relative to gases, and consequently the intermolecular distance in the liquids is much less than that in a gas. For example, at 0° C and 1 atmosphere pressure a gas occupies about 22.400 mL mol-1, while the majority of liquids occupy 10 to 100 ml. mol-1.
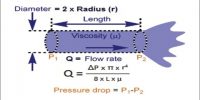
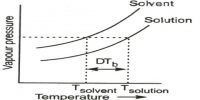
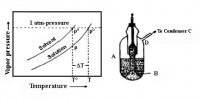
In a liquid the molecules are held close together by strong attractive forces. The molecules do, however, possess transnational motion in the liquid state. This motion acts against the attractive forces but it is not sufficient to overcome these forces. Because of this motion the molecules do not remain fixed in positions as will be found in a solid. These properties of the molecules in the liquid phase explain why a liquid has a definite volume but not a definite shape. Other characteristic properties of liquids such as vapour pressure, surface tension, viscosity etc., may also he explained in terms of the properties of the molecules.
The most familiar liquid states at room temperature are water, alcohol, benzene, carbon tetrachloride, corn oil, Castor oil, and gasoline.