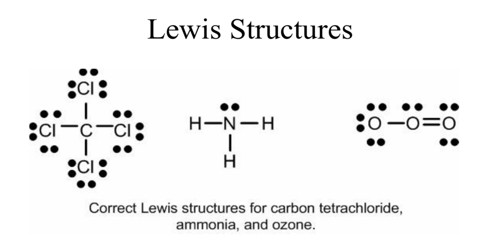
Lewis structures are very useful to predict whether ionic or covalent bonds will form between certain elements, and in what ratio they will combine. VSEPR predicts molecular shape well. However, they assume that pairs of electrons are all localized into bonds. Occasionally the true shape of a molecule (discovered by X-ray crystallography) shows that some electrons must be delocalized over more atoms.
The Lewis dot structure approach gives a superior idea of how the electrons are scattered within a molecule when dissimilar atoms bond together. Though, it cannot tell us the form of the molecule will be.
Lewis concept fails to explain:
- The cause of the covalent bond structure. It could not explicate the release of energy during the structure of a covalent bond.
- It could not clarify the shapes of molecules. The amount of enthalpy released during covalent bond formation.
- The nature of attractive forces between the constituent atoms of a molecule.
Resonance Structures
Sometimes a molecule or ion cannot be accurately represented by one electron-sharing Lewis diagram. E.g. carbonate ion CO3-.
Two or more resonance structures can be drawn, the true structure is a resonance hybrid (an imagined blending) of the different, non-existent resonance structures. X-ray crystallography shows that all three bonds are the same length.
The geometry of molecules containing covalent bonds. The structure of molecules such as PCI5, SF6, and IF7 in which the central atom has more than 8 electrons in its valence shell (violation of octet rule).
Lewis structures assume that electrons are either entirely located on one atom ( in ionic compounds) or shared equally between two atoms (covalent bonding). Ionic bonding and perfect covalency (perfect sharing) are two extremes. Most compounds are neither perfectly ionic nor perfectly covalent, but somewhere in between. The formation of molecules such as BF3, AlC13 in which the central atom has less than 8 electrons in its valence shell. Small highly charged cations (e.g. Mg2+, Al3+) can polarise the electrons in large anions giving covalent nature to some ionic compounds.