The Lewis theory defines an acid as an electron pair acceptor and a base as an electron pair donor. This theory broadened the scope of acid- base theory to include reactions that did not involve Ht. The Lewis Theory embraces many reactions that we might not think of as acid-base reactions. The reaction of boron trifluoride with ammonia is an example.
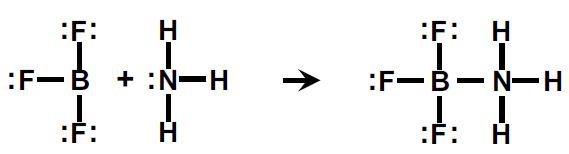
Boron tri-fluoride accepts the electron pair from the nitrogen atom of ammonia, so it is considered a Lewis acid. Ammonia donates the electron pair, so it is the Lewis base.
Strong acids
A strong acid is a substance that ionises completely in aqueous solution i.e. it exists in solution entirely as H3O+ and the conjugate base anion e.g. HBr (I) + H2O (I) → Br– (aq) + H3O+ (aq)
Other strong acids include:
– HCl: hydrochloric acid,
– HNO3: nitric add,
– H2SO4: sulphuric acid.
Similarly, a strong base ionises completely in aqueous solutions.
Weak acids
Most other acids and bases are weak. They are not completely ionised and exist in an equilibrium reaction with the hydronium ion and the conjugate base e.g. ethanoic acid is in equilibrium with the hydronium and ethanoate ions as follows:
CH3 COOH (aq) + H2O (1) = H3O + (aq) + CH3COO (aq)
The equilibrium lies well to the left; where only about 2% of ethanoic acid is dissociated.