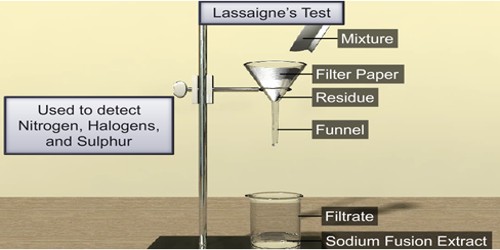
Lassaigne’s Test: Nitrogen, Sulphur, and halogens are detected in any organic compound by Lassaigne’s test. It is a general test for the detection of halogens, nitrogen, and sulfur in an organic compound. These elements are covalently bonded to the organic compounds. In order to detect them, these have to be converted into their ionic forms. This is done by fusing the organic compound with sodium metal. The aim of this test is to detect the halogens, nitrogen, and sulfur in an organic compound. In organic compounds, nitrogen, sulfur, and halogens are covalently bonded. Their detection in ‘Lassaigne’s test’ is possible if they are in the ionic form.
Principle: In Lassaigne’s test, the organic compound is fused with sodium to form ionic compounds & an aqueous solution is made. The aqueous solution obtained by extracting the fused mass in H2O— is called solution extract or Lassaigne’s extract/Stock solution. An organic compound containing C. H, N, S, X when fused with Na metal gives the following reactions:
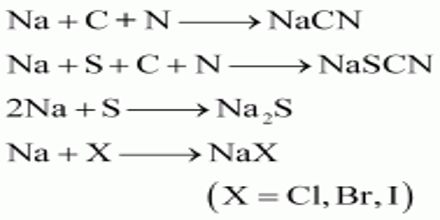
Why is Sodium (Na) used in Lassaigne’s test?
- It is highly reactive
- It is cheap
- The salts of Na are highly water-soluble
Estimation of S & P can be done together by the callus method.
Estimation of Sulphur:
- It is estimated by a modified Caries method.
- Estimation of Halogen: The amount of halogen person in an organic compound is estimated by the Carius method.
Test for Halogen:
Halogens present in an organic compound forms sodium halide on fusion with sodium metal. Sodium halide extracted with water can be easily identified by adding silver nitrate solution after acidifying with dil.HNO3.
If chlorine is present, a white curdy precipitate soluble in ammonium hydroxide solution is formed.
Na + Cl → NaCl
NaCl + AgNO3 → AgCl + NaNO3
Test for Nitrogen:
The carbon and nitrogen present in the organic compound on fusion with sodium metal give sodium cyanide (NaCN) soluble in water. This is converted into sodium ferrocyanide by the addition of enough quantity of ferrous sulfate. Ferric ions generated during the process react with ferrocyanide to form Prussian blue precipitate of ferric ferrocyanide.
Na + C + N → NaCN
6NaCN + FeSO4 → Na4[Fe(CN)6] (Sodium ferrocyanide) + Na2SO4
Na4[Fe(CN)6] + Fe3+ → Fe4[Fe(CN)6] (Ferric ferrocyanide)
Test for Sulphur:
If sulphur is present in the organic compound, sodium fusion will convert it into sodium sulphide. Sulphide ions are readily identified using sodium nitroprusside.
Na + S → Na2S
Na2S + Na2[Fe(CN)5NO] (Sodium nitroprusside) → Na4[Fe(CN)5NOS].