Phosphorus trichloride is a compound of phosphorus and halogen chlorine.
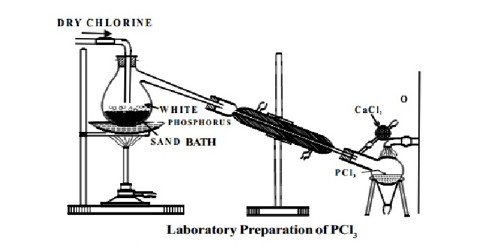
Preparation: Phosphorus Trichloride (PCl3) is prepared by heating white phosphorus in a current of dry chlorine.
P4 + 6Cl2 → 4PCl4
Dry white phosphorus is placed in the retort and gently heated on a water bath. A current of pure, dry chlorine is led over the phosphorus. The phosphorus trichloride formed being volatile distills over and is collected in a water-cooled receiver. The phosphorus dissolves into the phosphorus trichloride. In the laboratory, it may be more convenient to use the less toxic red phosphorus.
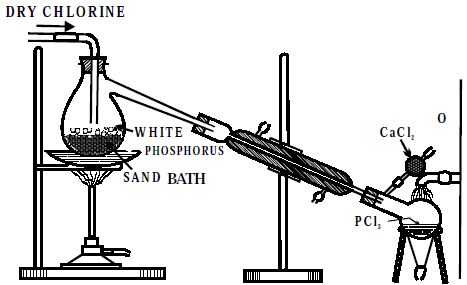
Fig: Laboratory Preparation of PCl3
The phosphorus trichloride obtained as above contains some PCl5 as an impurity. This is removed by distilling the PCl3 over white phosphorus. It appears as a colorless or slightly yellow fuming liquid with a pungent and irritating odor resembling that of hydrochloric acid. The reaction takes place in phosphorus trichloride to which elemental phosphorus is added. Chlorine is sparged into the phosphorus trichloride to react with dissolved phosphorus.
[It is generally removed from the reactor by distillation through the utilization of the heat of reaction.]
Physical properties
- Colorless low boiling liquid
- It fumes in moist air
- It has a pungent odor.
Chemical Properties
a. It is violently hydrolyzed by water giving phosphorus acid and hydrochloric acid gas.
PCl3 + 3 H2O → 3HCl + H3PO3
In a similar manner, it reacts with organic compounds containing hydroxyls (OH) group, such as acids and alcohols.
- PCl3 + 3CH3 COOH (Acetic Acid) → 3CH3COCl + H3PO3 (Acetyl Chloride)
- PCl3 + 3C2H5OH (Ethyl alcohol) → 3 C2H5Cl + H3PO3 (Ethyl Chloride)
b. It reacts with chlorine or sulphuryl chloride forming phosphorus pentachloride.
- PCl3 + Cl2 → PCl5
- PCl3 + SO2Cl2 → PCl5 + SO2
c. It readily combines with oxygen forming phosphorus oxychloride
2PCl3 + O2 →2POCl3
d. It reacts with SO3 to form phosphorus oxychloride and SO2
SO3 + PCl3 → POCl3 + SO2
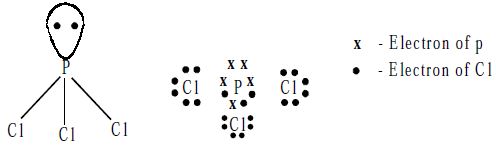
Structure: The PCl3 molecule has a pyramidal shape, which arises from the sp3 hybridization of the phosphorus atom. One of the tetrahedral positions is occupied by a lone pair of electrons.
Caution in Laboratory preparation –
- Causes severe burns to skin, eyes and mucous membranes,
- Very toxic by inhalation, ingestion and skin absorption,
- Reacts with water to evolve hydrochloric acid.
Usefulness:
Phosphorus trichloride is commercially the most significant compound and is used to prepare a wide variety of products, including soaps, detergents, plastics, and insecticides. Used for making pesticides, gasoline additives, textile finishing agents, germicides, medicinal products, and other chemicals.