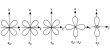
Phosphorus pentoxide P2O5 or P4O10 can be prepared by burning phosphorus with sufficient supply of air.
P4 + 5O2 →P4 O10
Physical properties
It is a white solid and an acidic oxide.
Chemical properties
- It reacts with moisture to form metaphosphoric acid.
P4O10 + 2H2O → 4HPO3
When the solution is boiled, the metaphosphoric acid is changed to orthophosphoric acid.
HPO3 + H2O → H3PO4
or, P4O10 + 6H2O → 4H3PO4
- Phosphorus pentoxide extracts water from many inorganic compound including sulphuric acid, nitric acid and several organic compounds. It is therefore, used as a powerful dehydrating agent.
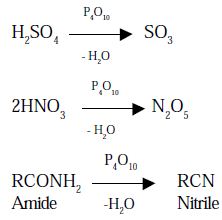
Use: It is used as a dehydrating agent.