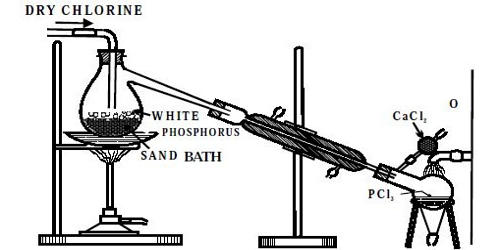
Phosphorus pentachloride is a pale greenish-yellow solid with the formula PCl5. It can be prepared by the action of dry chlorine on phosphorus trichloride. As per the law of mass action, Phosphorus pentachloride vaporizes almost without dissociation in an atmosphere of chlorine gas or phosphorus trichloride, the dissociation equilibrium is shifted to the left by the presence of the product.
Preparation: Phosphorus pentachloride (PCl5) is usually prepared by the action of an excess of chlorine on phosphorus trichloride. It can be prepared by chlorinating phosphorus trichloride with elemental chlorine.
PCl3 + Cl2 → PCl5
Reacting elemental chlorine with calcium phosphate, silica, and carbon at 1300°C will give phosphorus pentachloride. The reaction of sulfuryl chloride with elemental phosphorus will give phosphorus pentachloride. If thionyl chloride is used instead, phosphoryl chloride will also be produced. Burning phosphine in a chlorine atmosphere will produce PCl5.
Physical properties
- Phosphorus pentachloride is a yellowish-white crystalline solid.
- It sublimes on heating at 473 K and melts at 318 K under pressure.
Chemical properties
a. Phosphorus pentachloride dissociates on heating into phosphorus trichloride and chlorine.
PCl5 ↔ PCl3 + Cl2
b. It is violently hydrolyzed by water giving phosphorus oxychloride or phosphoric acid depending upon the quantity of water.
- PCl5 + H2O (insufficient of water) → POCl3 + 2HCl
- PCl5 + 4H2O (Excess of water) → H3PO4 + 5HCl
c. It reacts with compounds containing hydroxyl groups forming chloro derivatives. In all these cases, the hydroxyl group is replaced by chlorine.
C2H5OH (Ethyl Alcohol) + PCl5 → C2H5Cl (Ethyl Chloride) + POCl3 + HCl
d. It reacts with metals on heating to give corresponding chlorides.
2Ag + PCl5→2 AgCl + PCl3
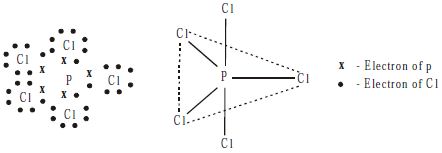
Structure
PCl5 molecule has a trigonal bipyramidal shape in vapor state which arises from sp3d hybridization of a phosphorus atom.
Uses of Phosphorus Pentachloride – PCl5
- Used as a chlorinating agent and catalyst ш making organic chemicals, intermediates, dyestuffs, etc.
- Used as a catalyst in the manufacture of acetyl cellulose the plastic film on which motion pictures are printed.
- Used as a chlorinating agent in organic chemistry.
- In the pharmaceutical industry, it is used in the manufacture of penicillin and cephalosporin.
- Used to produce acid chlorides and as a catalyst for cyclization and condensation reactions.