Phosphine (PH3) is the best-known hydride of phosphorus. It is created in the soil by the bacterial decline of phosphates. It is a compound which belongs to the group of organophosphorus compounds.
Laboratory preparation:
Calcium phosphide is mixed with water or dilutes HCl. This results in the formation of phosphine.
Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3
Ca3P2 + 6HCl → 3CaCl2 + 2PH3
In the laboratory white phosphorous is heated with concentrated sodium hydroxide solution in an inert atmosphere of CO2 to form phosphine.
P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2
It is usually obtained by boiling white phosphorus with 30-40% solution of caustic soda in an inert atmosphere of CO2.
P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2 (Sodium hypophosphite)
Phosphine so obtained is impure. It is passed into an aqueous solution of hydrogen iodide, PH4I is formed. PH4I is heated with KOH or NaOH, pure phosphine is obtained.
PH3 + HI → PH4I
PH4I + NaOH → PH3 + NaI + H2O
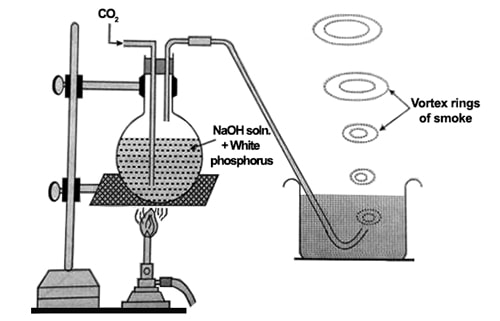
In this method, phosphine is prepared by hydrolyzing white phosphorus with a concentrated solution of sodium hydroxide.
Phosphine so obtained is impure. It is passed into an aqueous solution of hydrogen iodide, PH4I is formed. PH4I is heated with KOH or NaOH, pure phosphine is obtained.
Physical properties
- Phosphine is colorless gas with rotten fish odor.
- It is extremely toxic gas.
- PH3 is cautiously soluble in water and soluble in organic solvents.
- PH3 acts as a Lewis base by donating its lone pair of electrons when it reacts with hydrogen iodide.
- Under typical conditions, it is a non-ignitable gas. But, when you warm it, it bursts into flames, forming phosphoric acid.
- It explodes aggressively when we expose it to oxidizing agents.
Chemical properties
- Dissociation: Phosphine dissociates at about 723 K and gives red phosphorus.
4PH3 P4 →(23K)→ 6H2
- The action of air: It burns with oxygen and produces phosphorus pentoxide.
4PH3 + 8 O2 → P4O10 + 6H2O
Uses
- In semiconductor industries, it is used in small amounts as a dopant.
- PH3 is used in Holme’s signal due to its property of spontaneous combustion.
- Smoke screens
When PH3 burns it produces smoke which is dense enough to serve as smoke screens.
- Holme’s signal
Containers which have a perforated bottom and a hole at the top are filled with calcium phosphide and calcium carbide. These are thrown into the sea. Water enters the container through the bottom and reacts with calcium carbide and calcium phosphide to give acetylene and phosphine. Phosphine gets ignited spontaneously as it comes in contact with air and also ignites acetylene.
Thus a bright red flame is produced which is accompanied by huge smoke due to the burning of phosphine. This serves as a signal to the approaching ships.
- Ca3P2 + 6H2O → 2 PH3 ↑ + 3Ca(OH)2
- CaC2 + 2H2O → C2H2 ↑ + Ca(OH)2