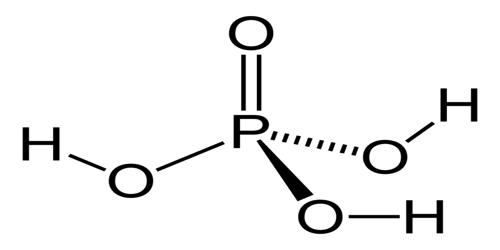
It is an acid-containing four atoms of oxygen, one atom of phosphorus, and three atoms of hydrogen. It is also known as phosphoric (V) acid or orthophosphoric acid.
Preparation
(1) Ortho Phosphoric Acid (H3PO4) is prepared by dissolving phosphorus pentoxide in water and boiling the solution.
P2O5 + 3H2O → 2H3PO4
(2) Laboratory preparation: In the laboratory, orthophosphoric acid can be prepared by boiling a mixture of red phosphorus with 50% nitric acid in a flask fitted with a reflux condenser on a water bath till no more oxides of nitrogen are liberated.
Iodine acts as a catalyst. The product is evaporated below 453 K and then cooled in a vacuum desiccator surrounded by freezing mixture when crystals of orthophosphoric acid are deposited.
P + 5HNO3 → H3PO4 +5NO2 + H2O
This fusion should be effected in a platinum crucible since phosphoric acid when heated to redness attacks either glass or porcelain. The acid if examined after this exposure to heat is found although its composition remains the same to have acquired new properties.
Physical properties
- It is a deliquescent crystalline solid.
- It is soluble in water. It cannot be obtained free from water.
- When exposed to a red heat and afterward cooled it forms a transparent brittle glass.
- In its liquid form, it appears as a clear, colorless solution and in its solid form, it appears as transparent, crystalline solid.
Chemical properties
- It is a tribasic acid. It combines with alkalies like NaOH to form three series of salts. This acid is a deliquescent solid, generally encountered as a viscous aqueous solution.

- On heating it gives pyrophosphoric acid at 523 K and at 589 K gives metaphosphoric acid
H3PO4 →(523K)→ H4P2O7 →(589K)→ 2HPO3 + H2O
- On reaction with silver nitrate, it gives a yellow precipitate of silver phosphate.
H3PO4 + 3AgNO3 → Ag3PO4 + 3HNO3
- It is weakly acidic, with three possible sequential deprotonation steps, forming phosphates. Like carboxylic acids, this acid can dimerize via a dehydration reaction to form phosphor anhydrides.
- As a result mono and disodium and potassium salts of phosphoric acid are routinely used as pH buffers.
- As with carboxylic acids, two phosphoric acid molecules may combine with the loss of water to form a diphosphate ester also referred to as pyrophosphate.
However, as phosphoric acid has further -OH functionalities triphosphates may also be formed. Salts of phosphoric acid are solid and many are relatively water-insoluble unless a strong mineral acid is present.
Uses
- It is used in the preparation of HBr and HI as a substitute for sulphuric acid.
- It is used as a souring agent in the preparation of soft drinks.
- It is used in the preparation of phosphate salts of sodium, potassium, and ammonium.
- It is used in the manufacture of phosphatic fertilizers.
- It is present in teeth and bone and helps in metabolic processes.