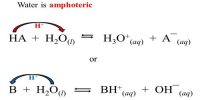
H2O and Kc are both constant and combining these constants together make up the ionic product constant for water, written KW.
KW = [H3O+] [OH-]
The value of KW is 1.0 x 10-14 mol2 dm-6 at 25°C. Like any equilibrium constant, KW varies with temperature.
These ions are produced in equal numbers in pure water, so
[H+] = [OH] x 10-14 = [H+]2
[H+] = [OH–] = 1.0 x 10-7 Mol.dm-3
Experiments have shown that the value for [H+] x [OH-] always equals 1.0 x 10-14 at 25°C even in solutions (such as acids or bases) where [H+] ≠ [OH-]. This means that if [H+] is known, then [OH-] can be calculated, or vice versa.
Example
Calculate the concentration of OH- ions in 0.10 M HCl
HC1 (aq) → H+ (aq) + Cl- (aq)
HC1 is a strong acid, so [H+ (aq)] will be 0.10 M.
Substituting [H+] = 0.10 into the KW expression, we get:
1.0 x 10-14 = (0.10) x [OH-]
[OH–] = 1.0 x 10-13 M