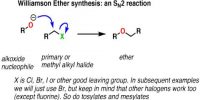
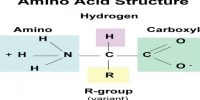
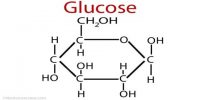
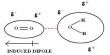
Intramolecular Hydrogen Bonding type of bond is formed between hydrogen atom and N, O or F atom of the same molecule. This type of hydrogen bonding is commonly called chelation and is more frequently found in organic compounds. Intramolecular hydrogen bonding is possible when a six or five membered rings can be formed.
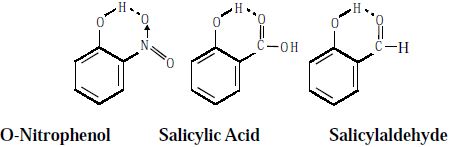
Intramolecular hydrogen bonding (chelation) decreases the boiling point of the compound and also its solubility in water by restricting the possibility of intermolecular hydrogen bonding.