Intermediate compound theory:
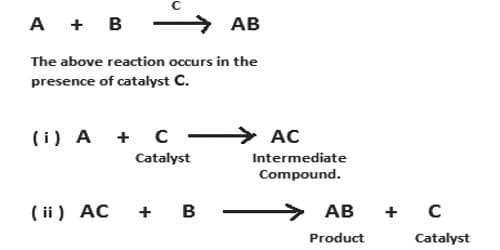
This theory was proposed by Clement and Desormes in 1806. The essential feature of the theory is that the catalyst chemically combines with one of the reactants to form an unstable active intermediate compound which then reacts further with the other reactants giving the final product and the catalyst is regenerated. Catalysis is the occurrence in which the rate of any reaction is distorted by the existence of an essence, which itself remains unchanged chemically in the reaction. The essence which alters the rate of the reaction is called a catalyst. According to this theory, the preferred reaction is brought about by a path involving the arrangement of an unsteady intermediate compound, followed by its disintegration into the preferred end products with the restoration of the catalyst.
In some reactions, the intermediate compound can be easily detected and even isolated whereas in some reactions the existence of the intermediate compound can be proved by sophisticated methods alone. A large number of catalytic reactions can be explained on the basis of this theory. Some examples are shown below:
(i) As shown earlier the oxidation of SO2 to SO3 by NO in the presence of oxygen or air is known to occur by the following sequence of reactions:
2NO + O2 → 2NO2
2NO2 + 2SO2 → 2SO3 + 2NO
2NO + 2SO2 + O2 → 2SO3 + 2NO net reaction
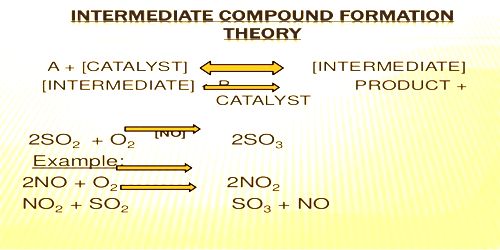
For example catalytic oxidation of SO2 to SO2 in the presence of NO as catalyst
2NO +O2 → 2MO2
NO2 + SO2 → SO3 + NO
This theory provides an explanation for the fact that catalyst remains unchanged in mass and chemical composition at the end of the reaction and its effectiveness even in little quantities. Many catalytic reactions can be explained on the basis of this theory.
However, in the absence of NO the oxidation is extremely slow. NO then may be looked upon as an “oxygen carrier”.
(ii) In the oxidation of iodides by hydrogen peroxide and the oxidation of iodides in the acid medium by oxygen, hypoiodous acid (or more correctly speaking IO–) is the intermediate active species that is responsible for the reaction.
H2O2 (aq) + I– (aq) → H2O (l) + IO– (aq)
H2O2 (aq) + IO– (aq) → H2O (l) + O2 (g) + I– (aq)
(iii) In the reaction between benzene (C6H6) and ethanoyl Chloride (CH3COCl) giving phenyl methyl ketone, C6H5COCH3; anhydrous AlCl3 is used as a catalyst.
C6H6 (l) + CH3COCl → C6H5COCH3 (l) + HCl (g)
AlCl3 first reacts with ethanoyl chloride giving rise to an intermediate species, CH3CO–, AlCl4– which then reacts with benzene and phenylmethyl ketone is formed. AlCl3 is regenerated.
CH3COCl + AlCl3 → CH3CO–.AlCl4–
The intermediate compound theory has been found useful in many reactions, particularly in homogeneous catalysis. The compound is formed with less energy consumption than needed for the actual reaction.
(iii) Acid catalysis of ester hydrolysis is another example where an intermediate is supposed to be formed. The case of hydrolysis of an ester such as methyl acetate in water may be considered.
CH3COOCH3 + H2O → CH3COOH + CH3OH
The reaction is slow but the presence of acid makes the reaction proceed at a much faster rate.
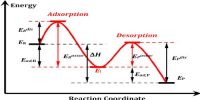
In simple words, we can say that a catalyst increases the rate of any reaction by providing an alternative pathway for the reaction to carry on with lower foundation energy.